See the Future
The first and only home ultrasound
for remote and reliable professional care
Pulsenmore (TASE:PULS) is a world leader in self-scan ultrasound devices for remote clinical diagnosis and screening. Our home ultrasound solutions improve access and continuity of care, providing efficiency that benefits clinicians and patients alike.
Improve resource management, productivity and workflow
Remote screening for earlier detection of warning signs
Increase patient convenience, satisfaction and engagement


Clinically Significant Remote Ultrasound
Self-Scan Device
A handheld ultrasound cradle that docks with the patient’s smartphone

Mobile App
Our user-friendly mobile app guides the patient through a self-scan and enables online consultation with a clinician


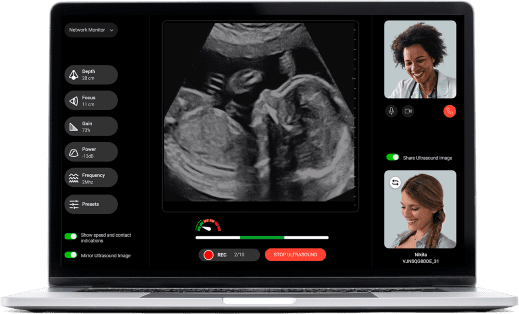
Clinician Dashboard
Using our cloud-based software, the clinician reviews scans and interacts with patients remotely
Software API
Enables integration with electronic health records




Self-Scan Device
The Pulsenmore handheld ultrasound cradle docks with the patient’s smartphone





Mobile App
Our user-friendly mobile app guides the patient through a self-scan and enables online consultation with a clinician



Clinician Dashboard
Using our cloud-based software, the clinician reviews scan and interacts with patients
Software API
Enables integration with electronic health records
COLLABORATIONS AND RECOGNITION


















WHAT EXPERTS ARE SAYING








Professor of Obstetrics and Gynecology










FEATURED NEWS
Major milestone! Pulsenmore secured a commercial agreement with Sheba Medical Center, Tel Hashomer for remote patient monitoring of high risk pregnancies.
Pulsenmore has received the TGA approval (the Australian Therapeutic Goods Administration)